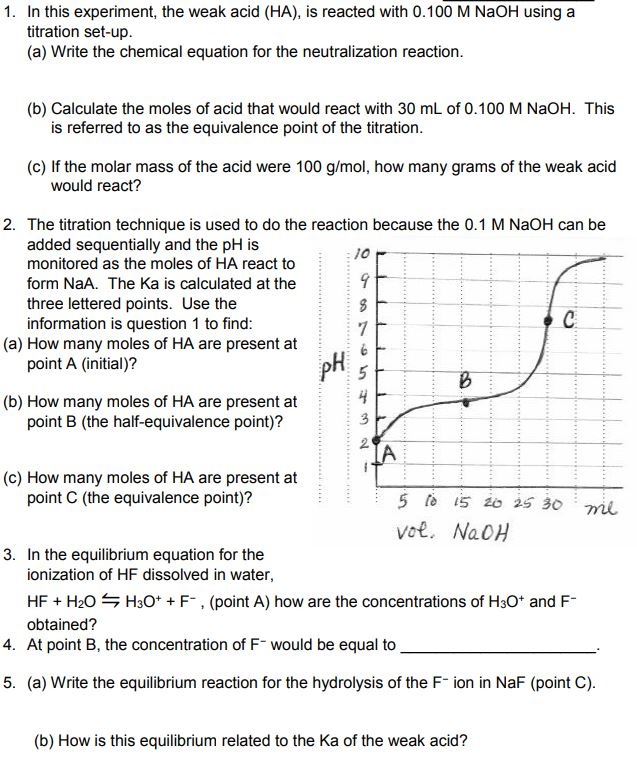
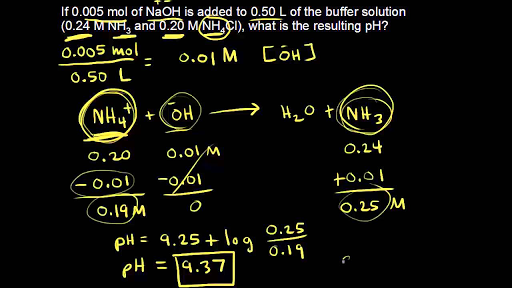
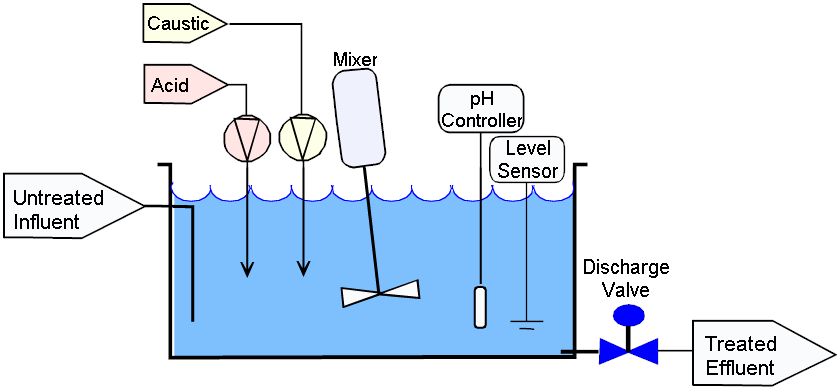
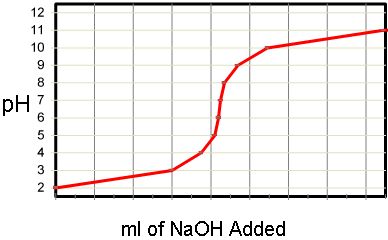
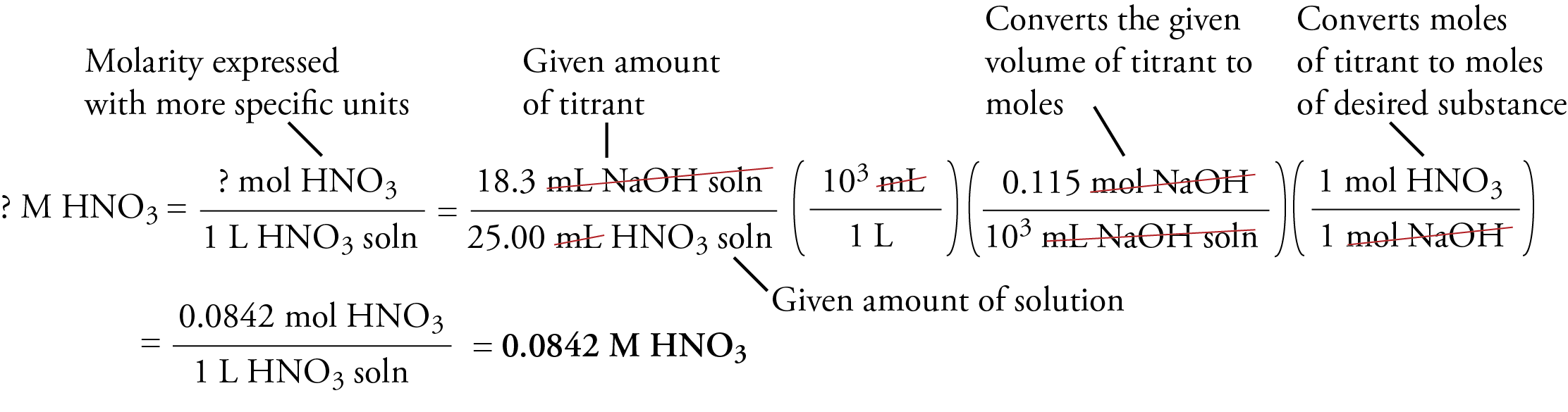
Calculate the concentration of HCl acid if 50 ml of HCl is required to neutralize 25 ml of 1 M NaOH in acid base titration.

Neutralizing Solutions with Sodium Hydroxide | Process & Chemical Formula - Video & Lesson Transcript | Study.com
What is the pH of a buffer that has 0.100 moles HC2H3O2 and 0.100 moles NaC2H3O2 in 1.00 l that has 0.010 moles NaOH added to it? - Quora