Dehydration Pathways of Gypsum and the Rehydration Mechanism of Soluble Anhydrite γ-CaSO4 | ACS Omega

Structures of a) gypsum, b) hemihydrate, and c) insoluble anhydrite... | Download Scientific Diagram

Dehydration Pathways of Gypsum and the Rehydration Mechanism of Soluble Anhydrite γ-CaSO4 | ACS Omega
A crystallographic study of the low-temperature dehydration products of gypsum, CaSOa ' 2H2Oz hemihydrate CaSOr ' 0.50H2O, and 1

Thermodynamic Modeling of Calcium Sulfate Hydrates in the CaSO4–H2O System from 273.15 to 473.15 K with Extension to 548.15 K | Journal of Chemical & Engineering Data

Table 1 from In Situ Raman Spectroscopic Study of Gypsum (CaSO4·2H2O) and Epsomite (MgSO4·7H2O) Dehydration Utilizing an Ultrasonic Levitator. | Semantic Scholar

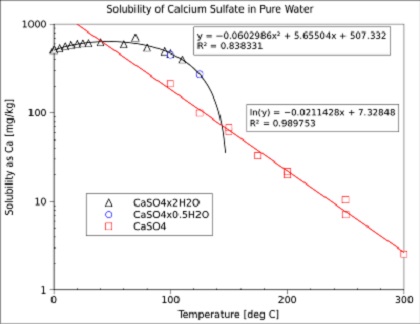
Determination and Analysis of the Solubility of CaSO4·2H2O and α-CaSO4·0.5H2O in Formamide Aqueous Solutions at T = 303.15–363.15 K | Journal of Chemical & Engineering Data

SEM of calcium sulfate (gypsum) precipitate in presence of additive... | Download Scientific Diagram
Membranes | Free Full-Text | Gypsum (CaSO4·2H2O) Scaling on Polybenzimidazole and Cellulose Acetate Hollow Fiber Membranes under Forward Osmosis

Dehydration Pathways of Gypsum and the Rehydration Mechanism of Soluble Anhydrite γ-CaSO4 | ACS Omega

Dehydration Pathways of Gypsum and the Rehydration Mechanism of Soluble Anhydrite γ-CaSO4 | ACS Omega
A crystallographic study of the low-temperature dehydration products of gypsum, CaSOa ' 2H2Oz hemihydrate CaSOr ' 0.50H2O, and 1

Dehydration Pathways of Gypsum and the Rehydration Mechanism of Soluble Anhydrite γ-CaSO4 | ACS Omega

Dehydration Pathways of Gypsum and the Rehydration Mechanism of Soluble Anhydrite γ-CaSO4 | ACS Omega